Metalosate® products are exclusive chelated minerals specifically designed for foliar application on plants. They are unique because the minerals are chelated with amino acids which are natural molecules found in all living things.
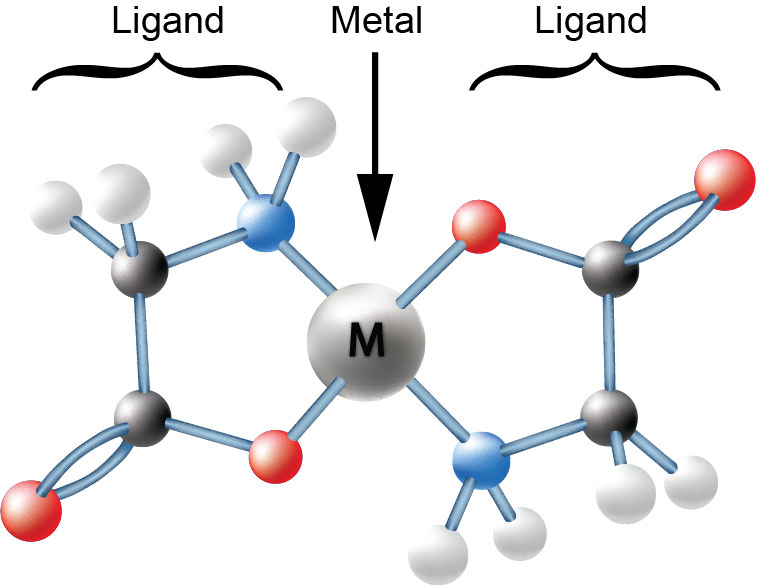
Chelation is the process of attaching a specific organic molecule, called a ligand, to a mineral ion at two or more sites to form a ring structure. Chelates can be either synthetic or naturally occurring. EDTA, DTPA, EDDHA and similar molecules are examples of synthetic chelating agents. Amino acids, hemoglobin, and chlorophyll are examples of naturally occurring chelates.
Solubility in water is essential for absorption by plants. This is true of the systemic chemicals as well as nutrients. Insoluble mineral salts, including all oxides, most hydroxides, carbonates and phosphates, and some sulfates cannot be absorbed by the plant.
When applying nutrients to the foliage, it is very important that the nutrients are in a form that is available to the plant. The surface of a leaf is negatively charged. When soluble forms of minerals are applied, most of the mineral ions (positively charged) are attracted to the negatively charged leaf surface and do not get into the vascular system of the plant. In order for the plant to translocate the minerals applied, they must be delivered to the vascular system.
By using an exclusive process of chelating the minerals with amino acids, Balchem has developed the optimal delivery system for plant mineral nutrition.
Minerals completely chelated with amino acids are neutral in charge. They are neither attracted to nor repulsed from the negatively charged surface of the leaf. Consequently, they freely pass through the cuticle. When the amino acid chelates reach the cell membrane, they are recognized by the mechanisms of absorption as a source of organic nitrogen. As a result, the entire amino acid chelate is taken into the cell very rapidly and efficiently.
Cell membranes do not have the ability to absorb synthetic chelates such as EDTA, DTPA, EDDHA, and others. For the mineral to be absorbed into the cell, these chelates must release the mineral. This leaves a vacancy in the chelate molecule displaying charges that must be satisfied. EDTA, for example, has a very high affinity for calcium. As a result, it will scavenge calcium from the surrounding environment, including cell walls and membranes. This can cause the collapse of the cell walls and leakage of the cell contents. This is the reason that the foliar application of high concentrations of EDTA often results in phytotoxicity.
Natural complexing agents, including some claimed to be chelators, such as lignosulfonates, humates, fulvates, and others, are actually very large and complex molecules. In addition, many products claiming to be amino acid chelates actually contain long chain hydrolyzed proteins. Because of their size, the likelihood of any of these molecules actually chelating a mineral is very low. Even if it does chelate, the size prevents it from passing through the cuticle. They do not offer the benefits of true amino acid chelated minerals.
The Metalosate products are available as calcium, magnesium, potassium, zinc, iron, manganese, boron, and copper packaged as individual chelated minerals. We also offer blends of nutrients in products such as Tropical®, Crop-Up®, Zinc Plus™, MZ™, and Multimineral®. We also offer an organic line of products which include calcium, copper, potassium, calcium-boron, crop up, magnesium, manganese, zinc, iron, and Multimineral.